Chemical Reactivity and `E^⊖` Values :
`=>` Transition metals vary widely in their chemical reactivity. Many of them are sufficiently electropositive to dissolve in mineral acids, although a few are ‘noble’—that is, they are unaffected by simple acids.
`=>` The metals of the first series with the exception of copper are relatively more reactive and are oxidised by `color{red}(1M)` `color{red}(H^+))`, though the actual rate at which these metals react with oxidising agents like hydrogen ion (`color{red}(H^+)`) is sometimes slow.
● For example, titanium and vanadium, in practice, are passive to dilute non oxidising acids at room temperature.
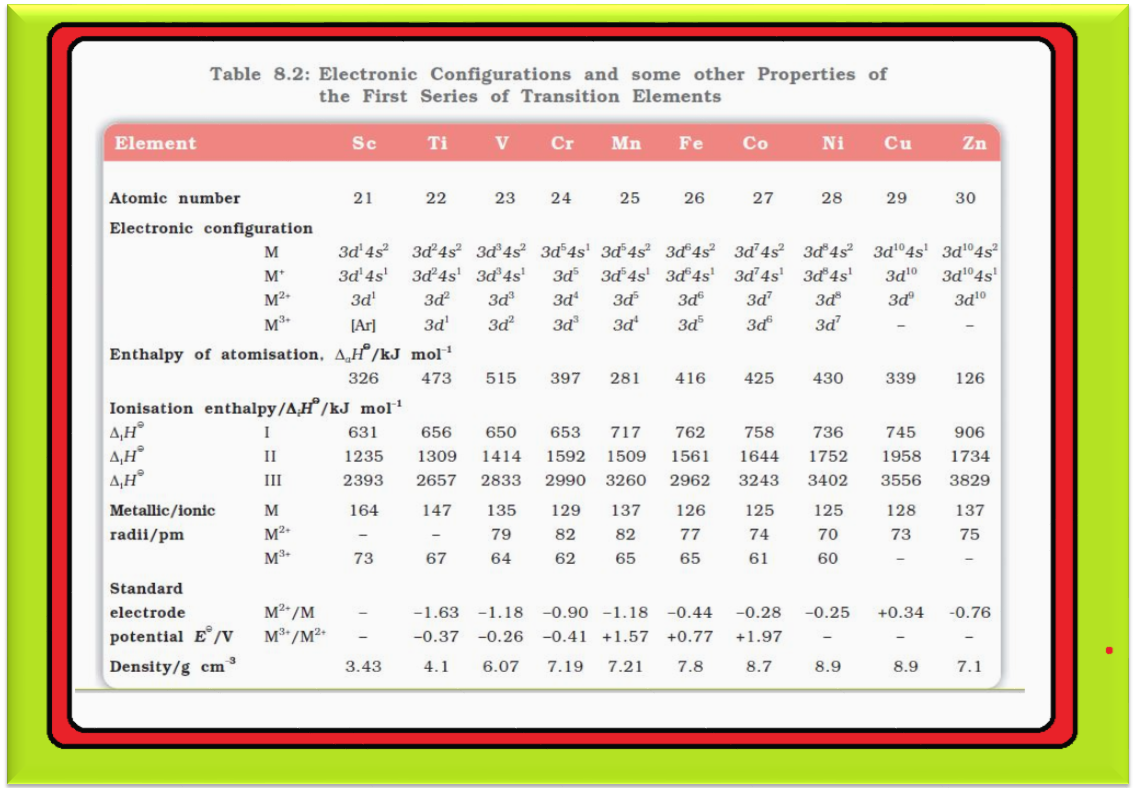
`=>` The `color{red}(E^⊖)` values for `color{red}(M^(2+)|M)` (Table 8.2) indicate a decreasing tendency to form divalent cations across the series. This general trend towards less negative `color{red}(E^⊖)` values is related to the increase in the sum of the first and second ionisation enthalpies.
`color{red}(text(Note ))` : (i) The `color{red}(E^⊖)` values for `color{red}(Mn)`, `color{red}(Ni)` and `color{red}(Zn)` are more negative than expected from the general trend.
(ii) Whereas the stabilities of half-filled `color{red}(d)` subshell (`color{red}(d^5)`) in `color{red}(Mn^(2+))` and completely filled `color{red}(d)` subshell (`color{red}(d^10)`) in zinc are related to their `color{red}(E^⊖)` values; for nickel, `color{red}(E^⊖)` value is related to the highest negative enthalpy of hydration.
`=>` An examination of the `color{red}(E^⊖)` values for the redox couple `color{red}(M^(3+)|M^(2+))` (Table 8.2) shows that `color{red}(Mn^(3+))` and `color{red}(Co^(3+))` ions are the strongest oxidising agents in aqueous solutions.
`=>` The ions `color{red}(Ti^(2+))`, `color{red}(V^(2+))` and `color{red}(Cr^(2+))` are strong reducing agents and will liberate hydrogen from a dilute acid, e.g.,
`color{red}(2 Cr^(2+) (aq) + 2 H^(+) (aq) → 2 Cr^(3+) (aq) + H_2 (g))`
`=>` The metals of the first series with the exception of copper are relatively more reactive and are oxidised by `color{red}(1M)` `color{red}(H^+))`, though the actual rate at which these metals react with oxidising agents like hydrogen ion (`color{red}(H^+)`) is sometimes slow.
● For example, titanium and vanadium, in practice, are passive to dilute non oxidising acids at room temperature.
`=>` The `color{red}(E^⊖)` values for `color{red}(M^(2+)|M)` (Table 8.2) indicate a decreasing tendency to form divalent cations across the series. This general trend towards less negative `color{red}(E^⊖)` values is related to the increase in the sum of the first and second ionisation enthalpies.
`color{red}(text(Note ))` : (i) The `color{red}(E^⊖)` values for `color{red}(Mn)`, `color{red}(Ni)` and `color{red}(Zn)` are more negative than expected from the general trend.
(ii) Whereas the stabilities of half-filled `color{red}(d)` subshell (`color{red}(d^5)`) in `color{red}(Mn^(2+))` and completely filled `color{red}(d)` subshell (`color{red}(d^10)`) in zinc are related to their `color{red}(E^⊖)` values; for nickel, `color{red}(E^⊖)` value is related to the highest negative enthalpy of hydration.
`=>` An examination of the `color{red}(E^⊖)` values for the redox couple `color{red}(M^(3+)|M^(2+))` (Table 8.2) shows that `color{red}(Mn^(3+))` and `color{red}(Co^(3+))` ions are the strongest oxidising agents in aqueous solutions.
`=>` The ions `color{red}(Ti^(2+))`, `color{red}(V^(2+))` and `color{red}(Cr^(2+))` are strong reducing agents and will liberate hydrogen from a dilute acid, e.g.,
`color{red}(2 Cr^(2+) (aq) + 2 H^(+) (aq) → 2 Cr^(3+) (aq) + H_2 (g))`